Does Citric Acid Conduct Electricity?
Follow Us:
Twitter
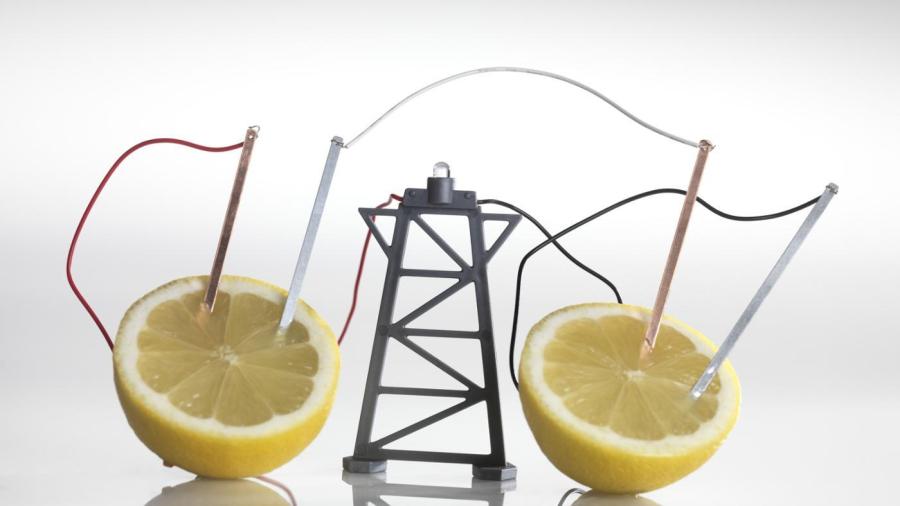
Citric acid is not a good conductor of electricity. This is because it is an example of a weak acid, which breaks down in water quickly.
Citric acid only produces relatively few ions, making it a weak conductor for electricity. On the other hand, strong acids conduct electricity very well. An example of a strong acid is hydrochloric acid. Strong bases and weak electrolytes also make good conductors. On the pH scale, citric acid is a 4, and hydrochloric acid is much stronger. Placing a strong electrolyte into a solution such as citric acid will increase its conductivity because solutions that contain large amounts of electrolytes produce more ions.





