How Do You Separate Sugar From Water?
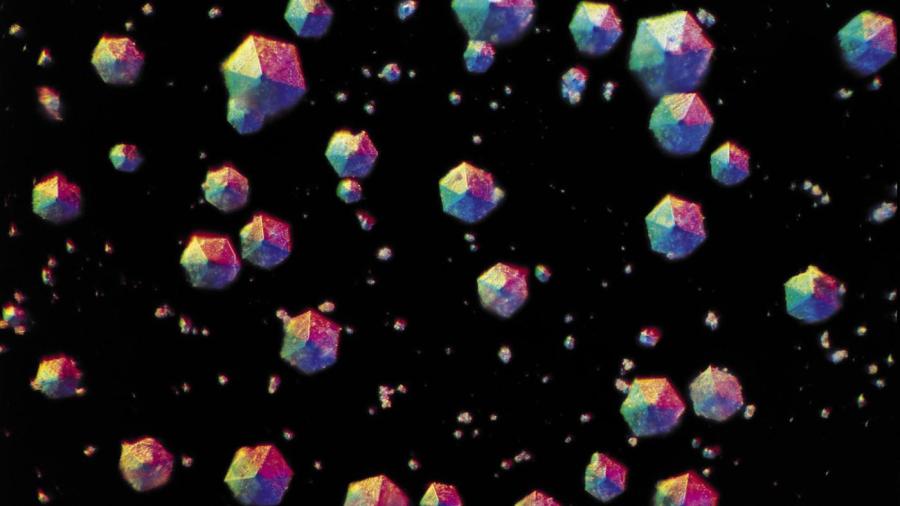
Sugar can be separated from a sugar and water solution through the process of crystallization. Crystallization is obtained by first turning the solution into a “supersaturated solution” through heating, and then cooling that supersaturated solution so that crystal formation can begin.
When sugar, or any other substance that’s soluble in water, is added to water, the substance breaks into incredibly small particles which are not visible to the naked eye. Water dissolves the solid substance, in this case sugar, and continues to do so until the solution becomes saturated.
Saturation of a solution is indicated by the point at which the solvent can’t dissolve any more of the solute. After a solution has reached saturation, it can be turned into a supersaturated solution by heating up the solution and adding more solute to the solution. Heating up a solution facilitates dissolving more solute in a solution than is possible at normal temperatures. After obtaining a supersaturated solution, it is possible to separate the solute from the solvent through crystallization of the solute. The process of crystallization is initiated when the solution starts to cool down.
As a supersaturated solution contains more solute than the solvent can ordinarily contain, it is very unstable and is highly conducive to crystal formation. To help begin the process of crystallization, only one or two crystals of the solute must be added to the solution as it cools down. This prompts the particles of the solute to separate from the solvent and start forming more crystals around the added crystals.





