What Is a Practical Application of Boyle’s Law?
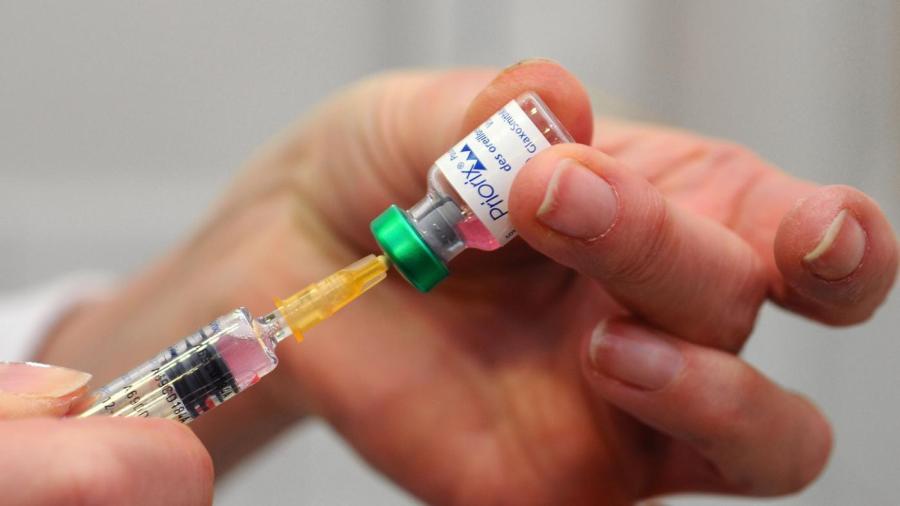
One practical application of Boyle’s law is drawing fluid into a syringe. Pulling back on the plunger increases the interior volume of the syringe and reduces its pressure. The fluid outside the syringe is sucked into the barrel until the interior and exterior pressure are balanced.
Boyle’s law states that the volume and pressure of a gas or liquid remain constant provided the substance’s temperature remains the same. This law, which was first declared by Robert Boyle in the 17th century, is vitally important in engineering applications such as engine design. Its formula is V1/V2=P2/P1 (at constant temperature), where V1 is the initial volume, V2 is the modified volume, P1 is the starting pressure and P2 is the modified pressure.
The law most closely related to Boyle’s law is Charles’ law, or the “law of volumes,” which is the work of French scientist Jacques Charles. It describes the relationship between volume and temperature. Both Boyle’s law and Charles’ law are gas theories that describe the behavior of a hypothetical “ideal” gas. In practice, however, both also apply to liquids. Boyle’s law is sometimes called Mariotte’s law, a reference to the French researcher who discovered the same principles 14 years after Boyle’s publication. This naming convention is popular in France and other European nations.





