What Factors Affect the Rate of Diffusion?
Rate of diffusion is influenced by several factors including temperature, concentration difference and particle size. The diffusion rate is also affected when there is a change in distance between the points where diffusion occurs.
Diffusion is the movement of atoms or particles from an area of high concentration to an area of lower concentration. This is also referred to as a change in concentration gradient. Concentration gradient is essentially the difference of molecule concentration within and outside of the cell. Rate of diffusion is directly proportional to the concentration gradient, which means that if molecular concentration is higher outside of the cell’s walls, molecules will move into the cell. This means that the concentration gradient is positive. A concentration gradient is considered negative when there are more molecules within the cell.
Particle Size and Diffusion Rate
Particle size is one factor that affects the rate of diffusion. At a given temperature, smaller particles will move more quickly than larger particles. This means that diffusion rate is inversely proportional to, or the opposite of, a particle’s size. A larger particle has less inertia, which means that it produces diffusion at a slower rate. However, temperature and particle size are directly proportional. As temperature rises, the rate of diffusion increases. Particles pick up speed at higher temperatures because more energy is available for them to use. Another explanation for this process is that temperature increases kinetic energy, which triggers the molecule to move at a faster pace. This, in turn, increases diffusion rate.
Influence of Viscosity on Diffusion Rate
Another factor that affects diffusion rate is viscosity. In a more viscous substance, which is one that is thicker, particles have difficulty moving. Therefore, they move at lower speeds than in a less viscous substance. For example, particles move more slowly in a solid substance like butter than in a less dense substance like water.
How Distance Affects Diffusion Rate
Variations in the distance between two points of diffusion also influences diffusion rate. When particles are closer together, there is less distance that molecules have to cover. This means that they have a higher diffusion rate. When particles are farther away, molecules take a longer time to travel between them, which results in a lower diffusion rate.
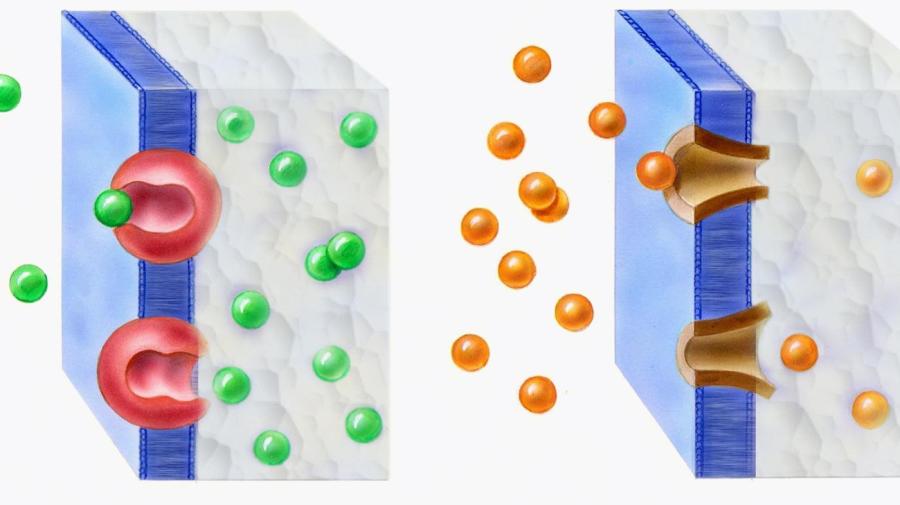
Diffusion through cellular membranes can take place with several substances. Ions, water and molecules that are required for basic cell functions can move in and out of cells through diffusion. Diffusion can take two forms, which are facilitated diffusion and simple passive diffusion. In facilitated diffusion, carrier proteins in cell’s membrane act as gatekeepers by regulating the type of molecules that move into and out of cells. During simple passive diffusion, molecules that are small enough can pass through the cell membrane’s lipid bilayer. Diffusion is affected by several factors, which include the diffusing molecules, properties of the cell and the environment around the cell.
Examples of Diffusion
Several common examples show diffusion at work in daily life. In plants, diffusion occurs in several ways. This includes roots taking in water and nutrients from the soil, water and nutrients being carried to all components of the plant, carbon dioxide diffusing into the plant’s leaves through the process of photosynthesis and a plant releasing oxygen through the stomata on its leaves.





